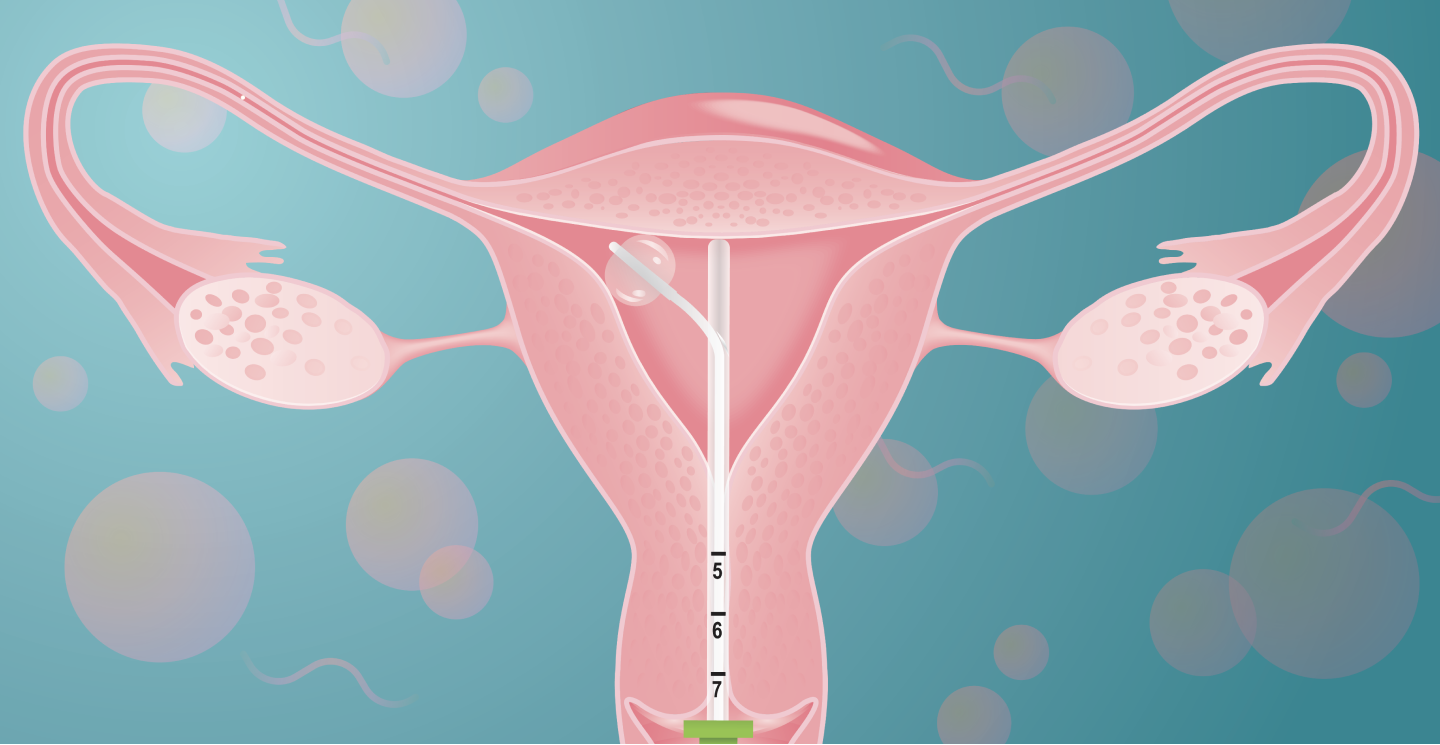
Clinical Data
Trial Design: Prospective, multi-center, unblinded pivotal clinical trial with historical control. Conducted under FDA Investigational Device Exemption (IDE) approval. (NCT0468847)
Top-Line Results1:
PRIMARY ENDPOINT
(for male-factor/unexplained fertility (1-20 million total motile sperm count, TMSC))
Pregnancy rate was 26.3% by subject (n=38) and 17.5% by cycle (n=57)
FemaSeed demonstrated a significantly higher pregnancy rate than the historical control
(6.7% pregnancy rate by cycle for intrauterine insemination (IUI) with male factor (greater than 1 million TMSC))
SAFETY
Adverse events were consistent with IUI.
1. Liu, J. H., Glassner, M., Gracia, C. R., Johnstone, E. B., Schnell, V. L., Thomas, M. A., Morrison, L., Lee-Sepsick, K. (2024). FemaSeed Directional Intratubal Artificial Insemination for Couples with Male-Factor or Unexplained Infertility Associated with Low Male Sperm Count. J Gynecol Reprod Med, 8(2), 01-12.
Market Research
Number of participants: 1,000 infertile women
More than 7 in 10 women were extremely or very likely to consider insemination with FemaSeed
For those who received IVF, 88% would have preferred FemaSeed prior to moving on to IVF
85% who received IUI would have preferred FemaSeed instead of IUI